A preparation method of a hindered amine light stabilizer and the intermediate
PATENT NUMBER: ZL201710408973.2
ABSTRACT
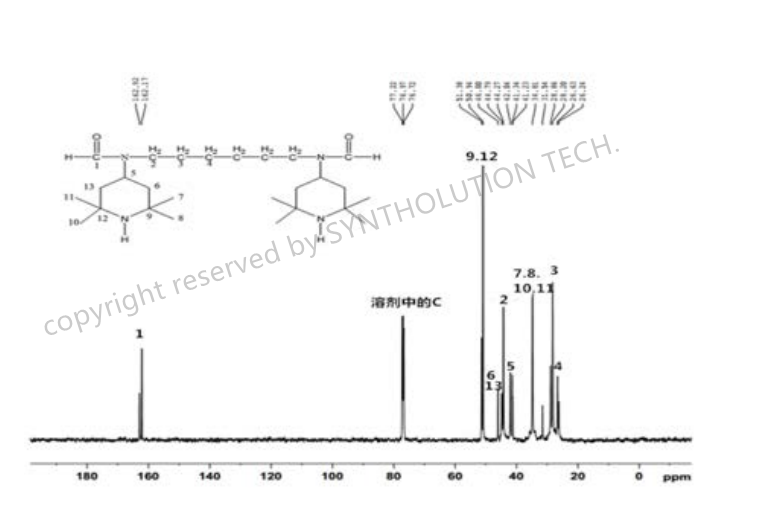
The preparation method of hindered amine light stabilizer N,N' -bis (2,2,6, 6-tetramethyl-4-piperidyl)-N,N '-diuronic alkyl diamine is as follows: the 4-formamide-2,2,6, 6-tetramethylpiperidine and dibromothane were stirred by reflux and catalyst for 1-24h, and water was added slowly immediately. The product was obtained after stirring, cooling, washing, filtering, and drying .
Among them, the preparation method of intermediate 4-formamide-2,2,6, 6-tetramethylpiperidine is: 2,2,6, 6-tetramethylpiperidine, formamide, Lewis acid catalyst, binding alkali agent, were put into heating reaction under atmospheric pressure for 1-24h. The product was obtained after stirring, cooling, washing, filtering, and drying.
The synthesis process of the invention is simple and easy to operate, and the post-treatment is convenient; the required raw materials, catalysts and solvents are easy to obtain. The whole process is in line with economic principles, safe and environmentally friendly, and the product yield is high.
A preparation method of hindered amine ultraviolet absorber
PATENT NUMBER: ZL201910576883.3
ABSTRACT
The invention discloses a preparation method of blocked amine uv absorber: mix malonic acid and pentamethylpiperidol, add toluene as solvent, add catalyst, and react at 100℃-110℃ for 6h. The reaction was stopped when the malonic acid content was lower than 1%. Drop the temperature to 50℃, add deionized water, stir for 30min, then add sufficient amount of cyclohexane with anisaldehyde, stir and dissolve, add piperidine and acetic acid, heat up to 70℃-80℃ for reaction, and separate the water generated in the reaction process. The reaction was stopped when the anisaldehyde content was lower than 1% in the liquid phase. The reaction solution was cooled to 5℃ and stirred for 1h until the solid was completely separated out. After filtration, the reaction was purified with 3 times the amount of ethanol and got the product.
The temperature of the hindered amine uv absorber in the preparation process is not higher than 110℃; the reaction adjustment is mild; the reaction process is easy to control; and the product purity is high; The early reaction system is not sensitive to water, and only needs to discharge the water in the system when the reaction is close to completion, which saves the operation process.


The product and the preparation method of a multi-effect thiether bisphenol acrylat antioxidant
PATENT NUMBER: ZL201910569404.5
ABSTRACT
Phenol acrylate multi-effect antioxidant molecules contains both main of hindered phenol antioxidant functional groups, auxiliary antioxidants thioether bond and carbon capture free radicals function of acrylic phenolic ester structure, which brings in the triple effect syncretic in both production and usage of polymer materials. This would have better protect polymer materials, avoid products of degradation and aging, solve the poor performance when single function antioxidant is used on polymer materials. Meanwhile, the corresponding preparation process reaction temperature and energy consumption is low, and by-product filtration can be removed without waste water,and recycling solvent, so the procedure is eco-friendly.
A preparation method for high purity pentaerythritol plastic auxiliary
PATENT NUMBER: 201910447473.9
ABSTRACT
The invention discloses a preparation method for a high purity pentaerythritol plastic auxiliary, in which the steps are as follows: mix functional components and pentaerythritol, heat up to 100-150℃ and stir for 15min; add dioctyl tin oxide, heat to 170-190℃ after nitrogen purge, continue the reaction until the liquid phase detection product content no longer increased; drop the temperature to 150℃, add xylene, continue to drop the temperature to 100℃, then add low molecular monic acid aqueous solution, stir at 100℃ for 1h, drop to room temperature; add methanol, stir for 3-5h, and the solid coarse product was filtered to obtain the pure product after re-crystallization with toluene.
After the reaction is completed, the low-molecular-monic acid was added directly into the reactant to carry out esterification reaction with the displaceable and trisubstituted impurities in the reactant, so as to dissolve the impurities and separate them from the product. The method does not need to use a large amount of solvents and multiple recrystallization operations, and has good impurity removal effect.
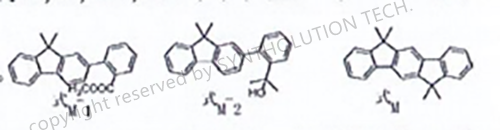
A preparation method of 6,6,12, 12-tetramethyl-6, 12-dihydroindene [1,2-b] fluorene
PATENT NUMBER: ZL201610332457.1
ABSTRACT
The invention discloses a preparation method of 6,6,12, 12-tetramethyl-6, 12-dihydroindene [1,2-b] fluorene.
The method includes: coupling reaction: methyl o-bromobenzoate is coupled with 9, 9-dimethyl su-2-boric acid to generate compounds as shown in Formula M-1; Addition reaction: the compound shown in Formula M-1 is added with methyl magnesium bromide, and then hydrolyzed to form the compound shown in formula M-2; Cyclization reaction: in the presence of acid, the compound shown in formula M-2 is cyclized and transformed into 6,6,12, 12-tetramethyl-6, 12-dihydroindene [1,2-b] fluorene as shown in formula M.
According to the preparation method of the invention, the process is simple and easy to operate; The raw materials needed are readily available at low cost; The yield is 77 ~ 88%, suitable for large-scale production.















